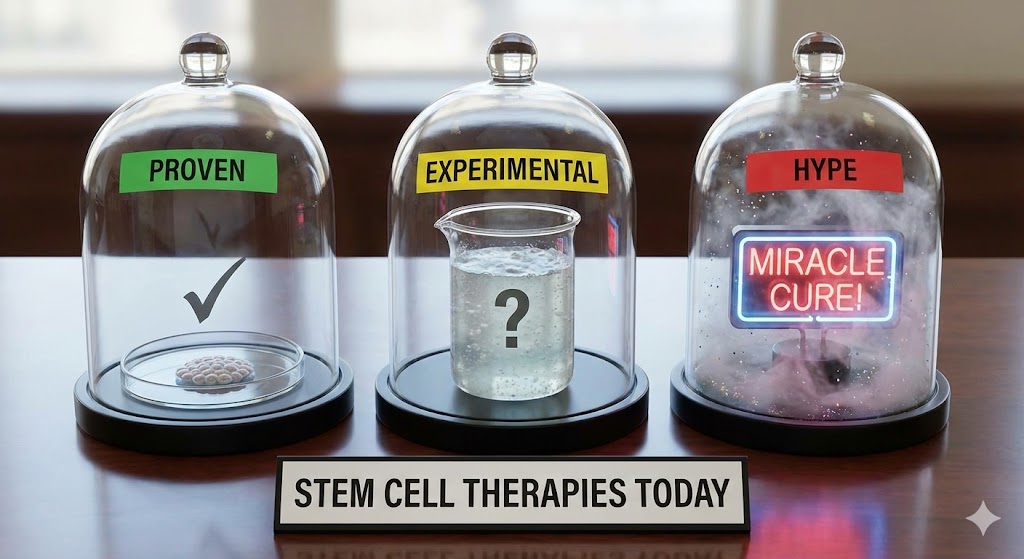
You’ve heard the buzz: injections of stem cells for joint pain, IV drips promising renewed vitality, cord-blood banking for the future. The promise is seductive — but which of these therapies actually stand on solid science, and which live in the shadowy zone between hope and marketing? This guide sorts fact from fiction. Stem cell therapy is real medicine in some cases. In others, it’s speculation dressed in white coats. Let’s break it down.
Understanding the difference could save you money, time — and possibly your health. It also helps you read the fine print when a glossy “regenerative medicine clinic” slides into your inbox.
Therapies That Are Proven: What Doctors and Regulators Accept
Decades of data support a small but powerful set of stem cell therapies. Chief among them is the hematopoietic (blood-forming) stem cell transplant — used for over 60 years to treat blood cancers, severe immune disorders, and bone marrow failure. In these cases, stem cells rebuild a damaged blood and immune system with safety protocols, donor-matching, conditioning regimens and long-term follow-up. This remains the gold standard of proven therapy in regenerative medicine.([nih.gov](https://stemcells.nih.gov/info/basics/stc-basics))
Umbilical cord blood stem cells also offer a legitimate, bankable alternative. Stored under strict conditions, cord-blood units can be used in place of bone marrow when a perfect donor match is unavailable — especially for pediatric patients or those of mixed ancestral backgrounds.([ncbi.nlm.nih.gov](https://www.ncbi.nlm.nih.gov/books/NBK536951/))
More recently, some cell therapies — using cultured mesenchymal stromal cells (MSCs) — have received regulatory approval for narrow, specific conditions. For instance, MSC-based therapy is now approved for graft-versus-host disease following transplant in certain pediatric patients.([reuters.com](https://www.reuters.com/business/healthcare-pharmaceuticals/us-fda-approves-mesoblasts-cell-therapy-graft-versus-host-disease-2024-12-18)) These cases illustrate that regenerative medicine is slowly broadening, but under tight regulatory frameworks.
Where Regenerative Medicine is Experimental — and When It’s Real Science
Beyond blood systems, the field is actively investigating whether stem cells can regenerate cartilage, repair heart muscle after heart attacks, heal spinal cord injuries, or reverse degenerative conditions. Many of these potential therapies remain in clinical trials — from early-phase safety studies to larger controlled trials testing efficacy.([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC9404248/))
For example, researchers are exploring MSC injections for osteoarthritis of knees and hips. Early-phase studies report reduced inflammation and symptomatic relief in some patients — especially when combined with rehabilitation. Nonetheless, large-scale, randomized controlled trials (RCTs) are still ongoing, and results remain mixed.([bmrat.org](https://bmrat.org/index.php/bmrat/article/view/79))
Similarly, experimental work on heart disease — using stem cells to regenerate cardiac tissue — remains at the preclinical or early-clinical stage. Some small trials have shown modest improvements in heart function, but long-term safety, functional recovery, and scalability remain significant challenges.([stemcellres.biomedcentral.com](https://stemcellres.biomedcentral.com/articles/10.1186/s13287-019-1165-5))
Where the Hype Outpaces the Science: Treatments to Approach With Caution
In the wellness world — especially among high-net-worth individuals chasing longevity or performance — clinics often pitch stem cells for a wide array of conditions: chronic pain, cosmetic rejuvenation, neurodegenerative disease, “immune boosting,” anti-aging, even sexual performance. These offers often mix real scientific language with marketing flair.
At present, most of these uses lack robust clinical data. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) have repeatedly warned consumers: outside approved uses or registered trials, stem-cell “therapies” may be unproven, unsafe, or outright fraudulent.([fda.gov](https://www.fda.gov/vaccines-blood-biologics/consumers-biologics/important-patient-and-consumer-information-about-regenerative-medicine-therapies))
Reported risks range from infection — due to unsterile processing or poor storage — to immune reactions and even tumor formation when uncharacterized cells divide inside the body.([pmc.ncbi.nlm.nih.gov](https://pmc.ncbi.nlm.nih.gov/articles/PMC9404248/)) Investing in such clinics without careful vetting may amount to buying hope — not healing.
How to Tell Legit Clinics from Smoke-and-Mirrors Clinics
When evaluating a stem-cell clinic or treatment offer, treat the process like due diligence on an investment. Ask yourself:
- Is the therapy FDA-approved or part of a registered clinical trial? Legitimate sites will openly share trial identifiers or approval status. If they don’t, that’s a red flag.
- Which type of cells are used? Cord blood, bone marrow, MSCs, iPSCs — each carries different regulatory, clinical, and safety implications.
- Is there published, peer-reviewed data backing up claims? Look for studies in major medical journals, not testimonials or before-/after photos.
- How transparent is the sourcing and processing? Cells should be harvested, processed, and stored under sterile GMP-grade conditions; follow-up and monitoring should be standard.
What Patients Should Realistically Expect (Today)
If you receive an approved stem-cell therapy today — say, a hematopoietic transplant — expect a rigorous process: screening, matching (if donor), conditioning chemo/radiation, possible side effects, months of immune system rebuilding. Success doesn’t look flashy: it’s gradual, careful, life-saving, not miraculous.
If you participate in an experimental therapy — say, an MSC injection for joint pain — think of it as a long-shot bet. You may feel improvement. Or you may feel nothing. Regeneration doesn’t happen overnight. You likely still need traditional rehabilitation, lifestyle changes, and realistic expectations.
Why Informed Skepticism Is Your Best Ally
In the world of regenerative medicine, hope and hype travel fast. Glossy brochures, luxury-clinic aesthetics, and exotic destinations blur the line between legitimate science and marketing spin. For wealthy, status-driven individuals, the promise of “repair,” “renewal,” “performance boost” can feel irresistible. But without evidence and transparency, chasing those promises can be dangerous — physically and financially. This makes skepticism not cynicism, but a strategic tool.
That doesn’t mean you ignore innovation. It means you approach innovation like a venture capitalist would: evaluate track record, assess risk, demand transparency, diversify — don’t put all your chips on the projection that the next big trial will succeed.
For those who value longevity, performance, and quality of life: treat stem cell therapy as an emerging asset class. Do the due diligence. Align with credible institutions. Demand data, not hype. That mindset turns hope into informed possibility.
What’s Next in the Series
Having laid out what’s proven, what’s experimental, and what to treat with caution, the next installment will dive deeper. We’ll explore the different sources of stem cells — cord blood, bone marrow, adipose, iPSCs — and how each origin shapes potential uses and risks. We’ll also look at how clinics structure protocols, handle processing, and attempt to standardize safety. Stay tuned.
Medical Disclaimer
This article is for informational purposes only and does not constitute medical advice. Stem cell therapies discussed here may not be approved for all indications in all countries. Always consult a qualified healthcare professional before making medical decisions or pursuing any treatment.
Related Articles
- The Hamptons Wellness Guide: Spas, Clinics and Retreats — a wider look at luxury health offerings on the East End.
- Longevity Lifestyle: How Hamptons Insiders Think About Healthspan — how affluent achievers structure their long-term wellness strategies.